Embryologische verklaring van Osteopathie, alles in het lijf is verbonden. De wetenschap achter osteopathie.
Een stuk tekst gepubliceerd in Nature, een wetenschappelijke onderbouwing van principes in de Osteopathie.
In het kort komt het erop neer dat alle structuren in ons lijf gevormd worden door groeikrachten tijdens en na de embryologische fase. Daarbij is beweeglijkheid en mogelijkheid tot vormverandering een essentieel punt. Kortom vrij vertaald: Alles moet beweging hebben en kennen voor een goede functie en groei, tijdens en zeker na de embryologische fase.
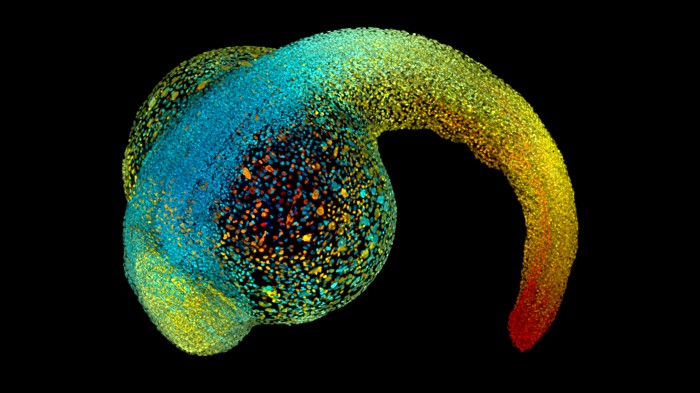
Developing embryos, such as this zebrafish, rely on physical forces to sculpt them as they grow. Credit: Philipp Keller/HHMI Janelia Research Campus
At first, an embryo has no front or back, head or tail. It’s a simple sphere of cells. But soon enough, the smooth clump begins to change. Fluid pools in the middle of the sphere. Cells flow like honey to take up their positions in the future body. Sheets of cells fold origami-style, building a heart, a gut, a brain.
None of this could happen without forces that squeeze, bend and tug the growing animal into shape. Even when it reaches adulthood, its cells will continue to respond to pushing and pulling — by each other and from the environment.
Yet the manner in which bodies and tissues take form remains “one of the most important, and still poorly understood, questions of our time”, says developmental biologist Amy Shyer, who studies morphogenesis at the Rockefeller University in New York City. For decades, biologists have focused on the ways in which genes and other biomolecules shape bodies, mainly because the tools to analyse these signals are readily available and always improving. Mechanical forces have received much less attention.
But considering only genes and biomolecules is “like you’re trying to write a book with only half the letters of the alphabet”, says Xavier Trepat, a mechanobiologist at the Institute for Bioengineering of Catalonia in Barcelona, Spain.
Over the past 20 years, more scientists have started paying attention to the importance of mechanics across a variety of developmental stages, organs and organisms. Researchers have begun to define the mechanisms by which cells sense, respond to and generate forces. They have done so by inventing bespoke tools and tricks, incorporating lasers and micropipettes, magnetic particles and custom-built microscopes. Most researchers are probing mechanical signals using cells or tissues cultured in a dish. But a few groups are studying whole animals, and sometimes they find different principles at work from those apparent in isolated tissues. These in vivo studies come with many challenges — such as measuring tiny amounts of force in complex tissues — but they are key to understanding the role of force in sculpting life, says Roberto Mayor, a developmental biologist at University College London.
As a handful of determined scientists begin to surmount those challenges, they’ve observed crucial forces shaping biology — from the earliest stages of an embryo’s existence to diseases that strike later in life. Down the line, this information might help scientists to design better interventions for problems such as infertility or cancer.
“Forces will operate in every single instance where shape is at play,” says Thomas Lecuit, a developmental biologist at the Developmental Biology Institute of Marseille in France.
Forceful from the start
Before an embryo can take shape, it has to break the symmetry of the smooth ball of cells. Having begun to understand the genetic and chemical controls over this process, scientists are now gaining more insight into the mechanics. “Little by little, a whole picture of the role of mechanical forces in development is appearing,” says biologist Jean-Léon Maître at the Curie Institute in Paris. For example, physical properties such as fluid pressure and cell density are key as the mammalian embryo creates its front, back, head and tail.
Maître’s group investigated how the initial ball of cells comprising the very early mouse embryo develops a large, fluid-filled cavity called the lumen. As this cavity fills, the cells that will become the fetus are pushed together on one side. This first symmetry-breaking event ensures that the embryo implants into the uterine wall correctly, and also governs which side of the embryo will be the back and which the belly. What wasn’t clear was how the embryo creates and positions the lumen (see ‘Pressure to develop’).
Source: Ref. 2
When they imaged the process in detail, Maître’s team found something unexpected. “We saw these little bubbles, these little water pockets forming between the cells,” says Maître. “They are transient — you just miss them if you don’t image fast enough.” The fluid in these bubbles comes from the liquid surrounding the embryo1, forced inside by the higher concentration of water molecules outside. Next, the team saw water from the individual bubbles flow, probably through gaps between cells, Maître thinks, into a single big lumen.
The researchers confirmed how this was happening by looking at the proteins that span the gaps between cells, which make contact with each other to stick cells tightly together2. As the bubbles appeared, these adhesion proteins seemed to break as the cells were pushed apart. Cells with fewer adhesion proteins were easier to force apart.
This is the first observation that pressurized fluid can sculpt the embryo by breaking the links between cells, says Maître. Why would the embryo force cells apart in order to build itself? “It definitely seems inefficient and risky,” he says. His best guess is that this strategy evolved not because it’s the best solution to the problem, but because it is ‘good enough’. He hopes that further understanding of embryo mechanics, which the team is now studying in human cells, could help in vitro fertilization clinics to identify which embryos to implant for a successful pregnancy.
Later in development, embryos break symmetry in another direction, differentiating head from tail. Otger Campàs, a biological physicist at the University of California, Santa Barbara, tracked the process of tail growth in embryos of zebrafish (Danio rerio)3. His group measured the forces involved by injecting oil droplets loaded with magnetic nanoparticles into the spaces between cells. Then the researchers applied a magnetic field to deform the droplets, so they could measure how the tissues reacted to the push.
To push and pull on cells in this zebrafish embryo, scientists distort a magnetic droplet (yellow) with a magnetic field.Credit: Alessandro Mongera and Otger Campàs, UC Santa Barbara
They found that the tip of the growing tail was in a state that physicists call ‘fluid’ — the cells flowed freely, and when pressed, the tissue deformed easily. The farther the scientists got from the tail end, the more rigid the tissue became. “We knew that it was solidifying, but we didn’t know the mechanism,” Campàs recalls.
There was nothing in between the cells that would add stiffness — no molecules making a structural matrix — but when the researchers measured the spaces between cells, they found them wide open in the squishy tail-tip, but smaller closer to the head4. As the cells crowded together, the tissue solidified. Campàs compares the transition to coffee grains being packaged: the grains flow freely into a bag, but become so tightly packed that the filled bag feels like a brick. He plans to investigate whether this mechanism underlies the formation of other embryonic structures, such as limb buds.
Making hearts and minds
Once the developing embryo has mapped itself out, individual organs begin to form. “Fundamentally, we have a poor understanding of how any internal organ forms,” says Timothy Saunders, a developmental biologist at the National University of Singapore. (The one exception, he notes, is the gut.)
That’s beginning to change. Saunders’s group, for instance, examined heart formation in embryos of the fruit fly Drosophila. There’s a crucial event when two pieces of tissue come together to form a tube that will ultimately become the heart. Each piece contains two kinds of heart-muscle cell. The pieces must zip up correctly, pairing like with like, for a healthy heart to emerge. “We often saw misalignment that was then corrected,” says Saunders. “What’s causing the correction?”
It turned out to be a force from within the heart cells themselves. A protein called myosin II, a close cousin to the protein that makes muscle cells contract, was known to flow from the middle of each cell to its edge, back and forth, during the zipping-up process. Then-graduate student Shaobo Zhang — who is now gearing up for a postdoctoral position at the University of California, San Francisco — wondered whether the myosin might create a force that tugs on the paired-up cells, breaking any connection between mismatched types.
To test his theory, Zhang sliced the paired cells apart with a laser. The cells jerked away from each other, like a taut rubber band snipped with scissors. “We could see beautiful recoil,” says Saunders. But when the team sliced apart cells lacking myosin II, “it just goes, mmph, nothing happens”. The myosin, like fingers pulling a rubber band apart, was creating the force to tug on the connections from within5. Mismatched cells, their links broken, would then have another chance to find the right partners.
Simple cell proliferation can also signal cells to arrange themselves properly, as researchers at the University of Cambridge, UK, discovered in embryos of the clawed frog Xenopus. The team, led by physical biologist Kristian Franze, already knew that as the eye and brain wire up, the eye neurons send out their axons — long projections that neurons use to contact each other — along a pathway defined by the stiffness of the brain tissue. Eye axons follow softer tissues towards a central hub in the developing brain6.
To determine when and how that pathway forms, the team custom-built a microscope with which they could simultaneously watch the process in vivo as they measured the tissue’s stiffness with a tiny probe7. They saw the stiffness gradient appear about 15 minutes before the axons arrived to follow it, says Franze, who also directs the Institute for Medical Physics and Microtissue Engineering at the University of Erlangen-Nuremberg in Germany.
How is the gradient formed? As in the developing zebrafish tails, the stiffer tissue in the frog brains seemed to contain a greater density of cells. When the team blocked cell division in the developing embryos, the stiffness gradient never appeared — and the axons couldn’t find their way. Packing a space with cells seems to be a quick and effective way to guide the wiring-up of the nervous system.
Continuing pressure
Fully developed animals must contend with forces, too, as they continue to grow or cope with disease. For example, when a body expands, the skin will grow to cover it. Surgeons take advantage of this in breast reconstruction, where more skin is needed to cover the planned implant. First, they insert a ‘balloon’ and inflate it gradually with saline over a period of months, stretching the existing skin, until enough new skin has grown to be used in a second surgery.
But how do skin cells respond to that pressure and multiply? Stem-cell biologist Mariaceleste Aragona tackled the question as a postdoc at the Université Libre de Bruxelles in Belgium, working with Cédric Blanpain. She implanted a pellet of a self-expanding hydrogel under the skin in mice8. As the hydrogel absorbed fluid, to a final volume of 4 millilitres, the skin stretched around it. Within a day of implanting the hydrogel, Aragona saw stem cells under the skin’s outer layer begin to multiply, providing the raw material that could differentiate into new skin.
But not all the stem cells proliferated in response to this stretching. Only a subpopulation, previously undefined, began pumping out new stem cells. “We still don’t know why,” says Aragona, now at the University of Copenhagen. Understanding this system might lead to methods to promote skin growth for surgical reconstruction or wound healing, adds Blanpain.
The mechanical properties of tissues also play a part in abnormal cell growth, such as in cancer. “Solid tumours are stiffer than normal tissues,” says Trepat. In part, that’s because of an excess of a fibrous meshwork called extracellular matrix around the cells, and also because the cancer cells themselves are proliferating, he says.
“Stiffness makes cancer cells more malignant,” Trepat adds, and if scientists could understand why, he says, they might be able to design treatments that change those physical properties and make cancers less dangerous.
In a related study, researchers at the Rockefeller University have identified mechanical forces that explain why some skin cancers are benign, and some malignant. Skin stem cells give rise to two different types of cancer: basal-cell carcinoma, which doesn’t spread beyond the skin, and invasive squamous-cell carcinoma. Each presses down on the underlying basement membrane, a layer of structural proteins that separates the skin’s outer layers from the deeper tissue. The benign basal-cell tumour rarely breaks through the basement membrane, but its more-aggressive counterpart often escapes to cruise the vasculature and lodge in other parts of the body (see ’The mechanics of skin cancer’)
Working with skin from mice, stem-cell biologists Elaine Fuchs and Vincent Fiore discovered that the benign cancer built a thicker, softer basement membrane, which contained the tumour cells like a glove as they pressed downwards. But the aggressive tumour fostered a thinner basement membrane.
A force from above also helped the invasive tumours to escape. Squamous-cell carcinomas build a stiff layer of differentiated skin cells called a keratin pearl. By pressing on the carcinoma’s top, the pearl helps the tumour to burst through the fragile basement membrane like a fist through glass9.
Before this work, says Fuchs, researchers had assumed that differentiated skin cells, those with fixed identities, couldn’t produce mechanical forces. “That’s, I think, the main surprise,” she says.
Next, Fuchs and Fiore plan to investigate how the cells perceive these mechanical forces, and how they convert the force into a program of gene expression that might produce more basement membrane or promote differentiation.
That question — how forces and genes are linked — is key, says Alan Rodrigues, a developmental biologist at the Rockefeller University. And it’s not just an issue for skin cancer. “The deep question in mechanics is actually thinking about how it relates to molecules,” he says.
Others, too, are investigating this link. “It’s not just, you know, ‘genes do everything’ or ‘mechanics does everything’,” says Lecuit. “It’s going to be an interesting dialogue between the two.”
Nature 589, 186-188 (2021)
References
- 1.
Schliffka, M. F. et al. Preprint at bioRxiv https://doi.org/10.1101/2020.09.10.291997 (2020).
- 2.
Dumortier, J. G. et al. Science 365, 465–468 (2019).
- 3.
Serwane, F. et al. Nature Methods 14, 181–186 (2017).
- 4.
Mongera, A. et al. Nature 561, 401–405 (2018).
- 5.
Zhang, S., Teng, X., Toyama, Y. & Saunders, T. E. Curr. Biol. 30, 3364–3377 (2020).
- 6.
Koser D. E. et al. Nature Neurosci. 19, 1592–1598 (2016).
- 7.
Thompson, A. J. et al. eLife 8, e39356 (2019).
- 8.
Aragona, M. et al. Nature 584, 268–273 (2020).
- 9.
Fiore, V. F. et al. Nature 585, 433–439 (2020).


